A client recently asked me about shockwave therapy in the hopes of speeding up the healing process. Shockwave isn’t something I knew much about, so I told them I’d delve into it and see if I came across anything worthwhile.
I started with Wikipedia to get a broad sense of the topic. There isn’t much on the Extracorporeal Shockwave Therapy (ESWT) page. (Extracorporeal is a reference to how the treatment is given outside of the body. Like rubbing the machine’s instrument on your leg.) Wikipedia told me what’s often the case with biomedical treatment devices, the medical value of shockwave therapy is “disputed.”
Wikipedia referenced a 2013 review declaring a benefit of shockwave treatment for plantar fasciitis. It also told me ESTW is approved by the FDA for treating plantar fasciitis and tennis elbow, but that was all. I figured I’d start with what shockwave seems to help the most with, plantar fasciitis. When a research review has been done on a topic, there’s normally a plethora of research on that topic.
Note: It’s important to realize the rest of this will deal with plantar fasciitis. This article is not about shockwave for every ailment in the body. It’s fair to call this a mental shortcut on my end. From my brain: If shockwave ends up being beneficial for plantar fasciitis, then it could very well have other applications. However, because at this stage shockwave is only approved for use with plantar fasciitis and tennis elbow, if shockwave ends up not being beneficial for plantar fasciitis, or the research isn’t that clear despite the FDA approval, then it’s likely shockwave isn’t worth much for anything else either. HOWEVER, that’s an estimate. You never know until you test. Plantar fasciitis is very different than say, a broken bone. This is an important point for all of us to keep in mind.
I find the full text of the review,
The first thing I always do with biomedical device papers is immediately look at who funded the study. I really don’t like to be so cynical as to say if a study on a device is funded by the people who make that device, then the study is useless, but you can’t not take this into consideration. In the least, a study by the device maker can be used as an indication independent research and money should be used to investigate as well.
As the client who asked me about this put it,
“It always seems the research on these sorts of things starts out so promising, then eventually ends up being far more equivocal.”
The reason for this is often funding. Device makers, or drug makers, fund the research -> A benefit is found -> Independent researchers delve in -> No benefit found -> Who are you going to believe?
When you only have research done by the device maker, or you have independent research conflicting with the device maker, then you have significant concerns. There is also what I call the “hyperbolic factor.” I can’t put a number on it, but when I see a study where the numbers jump out as too good to be true, that’s another flag. Again, tough to quantify this, but you often know it when you see it.
-> One way I look at this is if the value of the device, or surgery, is so far beyond what exercise and lifestyle modification gives, then that’s one indication it’s likely too good to be true.
-> Another way is if one study finds significantly greater benefits than other studies, that’s a good sign something odd is going on. I wrote about this with NSAIDs and surgery, regarding some papers which not only found greater benefits, but the studies were so clearly better conducted than others (randomized, placebo, all that good stuff), that I got seriously confused. Eventually I googled the researcher’s name…and found out he went to jail for making up the research.
Surprisingly, the review starts off under the abstract with:
“Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that all investigations were conducted in conformity with ethical principles of research.”
By the way, why is this conflict of interest disclosure always in small print? And sometimes it’s in the beginning, other times it’s at the end? This statement should probably be before the abstract, in a font larger than anything else in the paper.
Anyways, there’s no conflict funding wise. Great. I can delve into this and look more at the nitty gritty of the studies.
The review makes things even better by only looking at randomized controlled trials. Because of this, despite 136 articles qualifying based on keyword search, narrowing these 136 by prospective (looking forward; not backward) randomized controlled trials brought the number of studies reviewed down to only seven. The idea here is we have a lot less studies to look at, but they should be very good studies.
The next thought I have with these types of studies is, “You don’t have a placebo.” This is a tough thing research wise. With many modalities -icing, wrapping, massage therapy- you can’t do a sham treatment. A person knows whether their leg is cold, wrapped, or being rubbed. And they probably have biases one way or another to those sorts of things. I had the same thought with shockwave. You know if something is vibrating on your body. Yet consistently the authors mentioned these studies used placebo groups. I couldn’t figure out how though. How do you fake or hide this treatment? You can’t give a sugar pill.
Because the review doesn’t address this, after reading the review I decide to go into a specific study, so I can read the methods section and learn how a placebo is done with this research. The methods section is the one most don’t like to read, but often the most important.
–
Out of the seven studies in the review, I choose this one because it’s one of the more recent ones:
“This double-blind, randomized, placebo-controlled trial…”
Not only does this study use a placebo group, it’s also double blinded! “How the fuck did they do that?” Double-blind means the person giving the treatment and the person receiving the treatment don’t know what the treatment is. How do you not know you’re giving someone shockwave therapy??? Now I’m really intrigued.
“Patients in the control group received identical placebo intervention with a placebo hand-piece that prevented transmission of shock waves. The placebo hand-piece was identical in design, shape, and weight to ensure that there was no way to identify the placebo hand-piece.”
After reading this I still wasn’t quite sure how the placebo or double-blinding was being done. If you’re preventing the transmission, can the person still feel anything? I feel like it would certainly be less than without the hand-piece, no? And if it’s less, does that really still qualify as a solid placebo? How does the practitioner not know there is a piece on the device blocking transmission?
“The treatment in the placebo group was the same compared with the active one. Thereby, set up and sound created by the shock wave device was identical in both groups; however, no energy was administered in the placebo group. The intervention was performed in the office by the non-blinded orthopaedic surgeon or podiatrist,”
So we’ve gone from playing with the word placebo to playing with the word blinded. I don’t know if perhaps there are some unwritten rules in the research world as to what qualifies as blinded, but as best I can remember, and according to merriam-webster, and any other source I’ve looked at:
If the person administering the treatment knows what the treatment is, that’s not double-blinded.
Other things rubbed me the wrong way with this study too. I didn’t enjoy this graph:
This is an old statistics trick. You make two separate graphs look similar, yet use massively different scales. A quick look at the above and you’d think the placebo group (B -lower graph) had just as much, if not more, pain / discomfort during treatment, despite no treatment being done! (According to the authors.) That the placebo group had more reddening, swelling, and numbness. This is also despite the fact shockwave is known to cause pain and discomfort.
Yet a closer look at the graph and you realize despite the same bar size (the placebo actually looks as tall, but wider), only seven people in the placebo group had pain, yet a whopping 50 did in the shockwave. Same bar size; massively different numbers. Here’s more what the thing should have looked like. Black is shockwave; orange is placebo:
And then that moment came. I get to the end of the paper and see this:
What does everybody think Electro Medical Systems manufactures?
I decide to take this study with a grain of salt. I figure I’ll look into another one. That’ll give me a better chance to understand this whole funky placebo / double-blind thing going on here too.
–
Next up is the most recent study of the seven in the review:
–Chronic plantar fasciitis treated with two sessions of radial extracorporeal shock wave therapy.
First thing I do? Look at the funding.
“No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.”
Ah! Just when I thought I was playing CSI. Research wise, this is beautiful though. On to reading, with a particular interest in the methods section.
“Therefore we tested the hypothesis in the present prospective, randomized, double-blind, placebo-controlled study that treatment of chronic PF [plantar fasciitis] with two RSWT [shock wave] sessions…”
This one is also double-blinded. Great. How did they do it?
“Both patients and the study investigators were blinded for the entire duration of the study. Specifically, the study investigators did not have access to the patients’ treatment records, including patient allocation or the allocation sequence, until all patients had completed the 24-weeks of followup.”
Ok, so again we’re playing with the word “blinded.” Because so far, the doctor / person giving the shockwave therapy knows what treatment is being given.
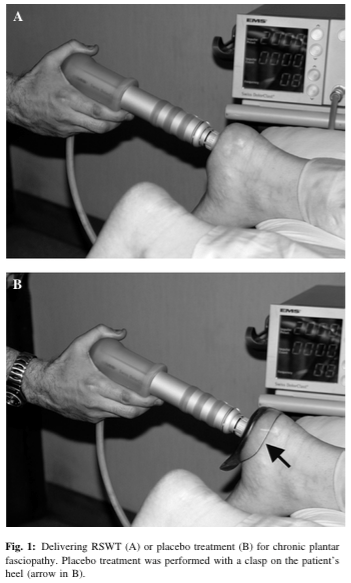
“Placebo treatment was performed identically but with a clasp on the heel that prevented transmission of the impulses from the applicator to the skin at the treatment site (Figure 1B)[…] The patients were not aware whether they received RSWT or placebo treatment.”
Not only are we playing with the word blinded, but we’re again playing with the word placebo. The fact of the matter is these two photos are not identical.
That doesn’t mean it affects the study, but you can’t say “well blah blah things were identical except blah blah.” Don’t use the word placebo then! This would be like a massage therapist comparing touching someone’s bare skin to touching someone with a plastic cup on them. It’s unequivocally different.
Bolding mine:
“The principal investigator who applied the treatments was not blinded and interacted with study participants strictly in a standardized way irrespective of treatment allocation, preventing any behavior that could have indicated to the patients whether they received RSWT or placebo treatment.”
Halfway into the paper we finally learn what double-blinded actually means in the context of this paper. It means the study wasn’t double-blinded. Like the other. Wonderful. Not to mention there is no way to know whether the above is true. Just because the practitioner follows the same protocol for both doesn’t mean they do it in the same manner. You can say the same sentence, but in about a million different ways. You can rub the same area who knows how many ways. The attitude of the practitioner could change, how they interacted with the patient could change. The way they said “How are you today?” could have been different. Or the way they said “See you next time.”
Does this influence the study? Who knows? Which is ok. The placebo wasn’t identical. The double-blinded wasn’t blinded. That’s ok. Just say it. Be honest about the study’s shortcomings. We understand science, especially health science, is hard shit. So don’t try to make a study look better than it is. Because then we start getting suspicious. What are you hiding?
This is where I originally intended to discuss shockwave some more. But part of me couldn’t get over this last bit of the study:
I’m honestly not sure why, but something about the acknowledgments section threw up a red flag for me. Maybe it’s because there was no letters after these people’s names. It’s common to have another PhD; another academic lettering thrown in there. This was also where I was expecting some funding information to come from, but the word fund isn’t even in this paper. Nobody paid for this? “Eh, what the hell. Let me google Rocco DePace.”
Rocco De Pace works in…medical devices. Well, that’s interesting. What medical device company does he work for? After a brief amount of more digging, I come across this link: http://www.dolorclast.com/pdf/Press%20Release%20USA.pdf
Which tells me, “Rocco DePace, Managing Director for Electro Medical Systems Corporation…”
The same Electro Medical Systems that funded Radial Extracorporeal Shock Wave Therapy Is Safe and Effective in the Treatment of Chronic Recalcitrant Plantar Fasciitis. They funded one study; they had their managing director thanked for his “support and contributions,” whatever that means, in another study.
–
Oh, for fun, let’s look at another study in that review.
What’s the “new-generation pneumatic device”?
The “Swiss DolorClast.” Brought to you by Electro Medical Systems. While the study says no benefits or funds were received, the same device manufacturer was used again.
After seeing how EMS shadily had an influence on the other study, I’m skeptical of this one. However, this is ideally what we we’re looking for at this point. EMS device used, but independently. (No funding issues.) What’d this study find?
“It appears that a placebo effect was the most important independent factor influencing the final result after six months. We observed a significant decrease in VAS values in groups 0 and 1.”
No difference between placebo (0) and treatment (1) groups. More on this later.
–
Another study from the review:
While I wasn’t able to find out what the new electromagnetic shock wave device was in this study, I did notice it has two of the same authors as the first study I went over, Radial Extracorporeal Shock Wave Therapy Is Safe and Effective in the Treatment of Chronic Recalcitrant Plantar Fasciitis. Those two authors are L. Gerdesmeyer and H. Gollwitzer. (This is the study funded by EMS.)
These two have worked quite a bit together. Pubmed says 29 times. Gerdesmeyer 18 times on shockwave; Gollwitzer 11. Gerdesmeyer also wrote two books about shockwave therapy. Here and here on Amazon. I think it’s worth mentioning the latter book came out before these two studies I’m referencing. If you have a book written on a topic, then perform research that topic, and you don’t disclose an author of your study has a book written on the topic…
–
The next study of the seven reviewed I looked at was:
This study had the following disclosure:
“This multicenter, double-blinded clinical investigation, entitled protocol PF-01: A Comparative Randomized Placebo-Controlled Clinical Trial of OrthospecTM Versus OrthospecTM Placebo for the Relief of Pain in the Treatment of Proximal Plantar Fasciitis, was conducted under the United States Food and Drug Administration Investigational Device Exemption #G020175 to determine the safety and efficacy of the OrthospecTM ESWT device. The study sponsor, Medispec LTD, 12850 Middle- brook Road, Suite 1, Germantown, MD 20874, provided the extracorporeal shockwave therapy and plantar pressure assessment devices, and funded the investigation.”
Medispec is a company selling shockwave technology. Their product catalog garners a good chuckle. I didn’t know shockwave is apparently also used to treat erectile dysfunction. I wonder if it’s the shockwave that supposedly helps, or the way all the women in their product catalog look:
And at their booths (from their LinkedIn page):
To be fair, they do have this photo in there too. Sorry to anyone who has erectile dysfunction, but this is a hysterical photo:
In Los Angeles, I’ve heard there’s a place called Happy Endings that’s probably cheaper.
–
Next study in the review:
At the bottom:
Let’s transition back to the review itself. Remember, this is the study that looked at the seven others. Bolding mine:
“Speed et al. [20] concluded that there appeared to be no treatment effect of moderate-dose ESWT. Unfortunately, as the data presented in the paper were not comparable to others, it could not be included in the analysis. Of all the trials, it seems their trial was the only one not showing significantly favorable results after ESWT. There are two potential reasons for this. The followup of patients who had nonoperative treatment in their study was 3 months. Other studies included patients only if nonoperative treatment for 6 to 12 months failed (Table 2). Another potential reason is that the energy flux density administered in the study of Speed et al. [20] was the lowest of all the studies included. In a recent meta-analysis, Chang et al. [4] concluded that lower energy intensity shock waves are less effective.”
Wasn’t there another study though, that used an EMS device but was independent, which found no benefit? The authors of the review didn’t like this study due to, amongst other reasons, a small amount of patients being used. For now, let’s go with their rationale.
Out of the now six studies, according to the authors of the review, one did not find any benefit from shockwave therapy. That study is
–Extracorporeal shock wave therapy for plantar fasciitis. A double blind randomised controlled trial.
As I quoted above, the authors gave various rationale for why this study didn’t find any benefit. Having to wait a few extra months seems like an odd rationale. The other one is the dosage. That certainly makes sense to look into.
The other factor, the one the authors don’t mention? The one study finding no benefit is the one study of the six not funded by a medical device manufacturer, not having a managing director of a medical device manufacturer acknowledged, not having an author with financial interests. The one study finding no benefit was funded by an arthritis charity. The two studies of the seven finding no benefit had no financial conflict.
Why the authors of the review don’t mention this, I’m not sure. My assumption is they either didn’t look into it, or intentionally didn’t bring it up.
–
I looked into the authors of the review. One author, David J. Redfern owns his own shockwave machine at a hospital he works out of:
Maybe he had this before the doing the review. That would certainly be a conflict. Or maybe the review, since it casted a positive light on shockwave, caused him to buy one.
Another author, Matthew Solan, works with David Redfern.
One of the treatments that practice offers is shockwave therapy (here):
Based on pubmed, all of the researchers had not published on shockwave therapy before. So it’s not as if they have this long history of promoting it, like was found with some others.
I emailed two authors from the review, Dr. Adeel Aqil and Professor Justin Cobb. (I couldn’t find email addresses for the other authors.) I gave them a week to reply and never heard back. Here are three pictures of my email, which also provide a summary of everything above (I forgot to mention in the email the study not influenced by EMS, but using their device, found no benefit):
–
Closing out
- A meta-analysis narrowed the research on shockwave therapy and plantar fasciitis down to seven studies. In terms of being randomized, etc., these seven are likely the best studies on the topic.
- Of those seven, five had clear conflicts of interest. These five found benefits.
- Two of the seven studies did not find a benefit. These two studies had no conflicts.
- These two studies were not completely similar to the others (most research has this issue), and the authors of the review essentially dismissed them.
- The authors of the review are not in the clear conflict wise. I gave them an opportunity to do so, but did not hear back.
- These two studies were not completely similar to the others (most research has this issue), and the authors of the review essentially dismissed them.
- Shockwave therapy is painful. I’m not sure there is any modality I’m a fan of that puts people in pain to help them get of it. Barring the here and there pain of intense exercise (like lactic acid), I’m not sure there is any modality at all, which puts people in pain, which I’m a fan of.
- Remember my earlier note, this all dealt with plantar fasciitis. While I find it highly unlikely shockwave is going to have benefits for other ailments, especially after catching all these issues on the ailment shockwave is purported to have the most benefit, it can’t be ruled out. I think a fair answer to this post’s title, “Does shockwave therapy have merits?” is “probably not.”
–































Posted on May 1, 2015